Cesium

| Atomic Number: | 55 | Atomic Radius: | 343 pm (Van der Waals) |
| Atomic Symbol: | Cs | Melting Point: | 28.5 °C |
| Atomic Weight: | 132.9 | Boiling Point: | 671 °C |
| Electron Configuration: | [Xe]6s1 | Oxidation States: | +1, −1[3] (a strongly basic oxide) |
Symbol: Cs Atomic Number: 55 (protons in nucleus) Atomic Weight: 133 (naturally occurring) Radioactive Properties of Key Cesium Isotopes and an Associated Radionuclide Radiation Energy (MeV) Isotope Half-Life Specific Activity (Ci/g) Decay Mode Alpha (α) Beta (β) Gamma (γ) Cs-134 2.1 yr 1,300 β - 0.16 1.6.
History
- Notes on the properties of Cesium: Specific Heat: Value given for solid phase. Up to date, curated data provided by Mathematica 's ElementData function from Wolfram Research, Inc.
- Naturally occurring cesium consists entirely of the nonradioactive isotope cesium-133; a large number of radioactive isotopes from cesium-123 to cesium-144 have been prepared. Cesium-137 is useful in medical and industrial radiology because of its long half-life of 30.17 years.
From the Latin word caesius, sky blue. Cesium was discovered spectroscopically in 1860 by Bunsen and Kirchhoff in mineral water from Durkheim.
Sources
Cesium, an alkali metal, occurs in lepidolite, pollucte (a hydrated silicate of aluminum and cesium), and in other sources. One of the world's richest sources of cesium is located at Bernic Lake, Manitoba. The deposits are estimated to contain 300,000 tons of pollucite, averaging 20% cesium.
It can be isolated by elecytrolysis of the fused cyanide and by a number of other methods. Very pure, gas-free cesium can be prepared by thermal decomposition of cesium azide.
Properties
The metal is characterized by a spectrum containing two bright lines in the blue along with several others in the red, yellow, and green wavelengths. It is silvery white, soft, and ductile. It is the most electropositive and most alkaline element.
Cesium, gallium, and mercury are the only three metals that are liquid at room temperature. Cesium reacts explosively with cold water, and reacts with ice at temperatures above -116C. Cesium hydroxide, the strongest base known, attacks glass.
Uses
Because of it has great affinity for oxygen, the metal is used as a 'getter' in electron tubes. It is also used in photoelectric cells, as well as a catalyst in the hydrogenation of certain organic compounds.
The metal has recently found application in ion propulsion systems. Cesium is used in atomic clocks, which are accurate to 5 s in 300 years. Its chief compounds are the chloride and the nitrate.
Isotopes
Cesium has more isotopes than any element--32--with masses ranging from 114 to 145.
Donald B. Sullivan, a physicist and chief of the time and frequency division of the National Institute of Standards and Technology, explains.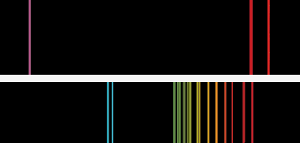
When the cesium second was defined in 1967, it was based on a measurement of the number of cycles of the radiation from a particular cesium-133 transition with reference to the second commonly used in civilian timekeeping, which at that time was based on astronomical observations. The objective was to improve the stability of timekeeping in a manner that would be invisible to the general population. The decision to redefine the second was ultimately that of the International Committee of Weights and Measures, an organization that works to standardize and coordinate measurements. At its 13th official meeting in 1967, the committee adopted the following definition: 'The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium-133 atom.'
In making this decision, the committee relied primarily on a measurement first reported in 1958 that compared the cesium transition frequency to the second of ephemeris time, which is defined by the orbital motion of the earth about the sun. A collaboration between the National Physical Laboratory (NPL) in England and the United States Naval Observatory (USNO) produced the measurement. Louis Essen of NPL had just developed the worlds first reliable cesium-beam atomic clock, and William Markowitz of USNO had developed a moon-position camera that provided a way to easily access ephemeris time, something that had been previously very difficult to do.
For years prior to this measurement people had recognized that the earths motions were not sufficiently predictable for highly accurate timekeeping, and alternatives based on atomic clocks were under study. Harold Lyons and his collaborators at the National Bureau of Standards (NBS) (now renamed the National Institute of Standards and Technology (NIST)) made one of the first accurate assessments of the cesium frequency relative to earth-based time in 1952. The cesium-beam standard used for this measurement could be operated for only short periods, so the uncertainty of Lyons measurement was too large for it to serve as the basis for a new definition.
Atomic Number 55
NPL holds the distinction of developing the first operational cesium-beam atomic clock. Essen received funding for his clock project in 1953 and had a very reliable version running within two years. In a collaboration between Essen and Markowitz, the relative durations of the astronomical and atomic (cesium) seconds were measured over an averaging time of 2.75 years with a final determination that the cesium frequency was 9,192,631,770 20 Hz. The definition of the second accepted internationally uses the exact number produced by this measurement. It is interesting to note that the timing comparison across the Atlantic Ocean was made using a method based on simultaneous reception of the shortwave time signals broadcast by the NBS radio station WWV, which was then located on the east coast of the United States.
Cesium Electron Number
The story of these measurements is nicely detailed in Splitting the Second: The Story of Atomic Time, by Tony Jones (Institute of Physics, 2000). The report on the measurement of the cesium frequency appeared in Physical Review Letters, vol. 1, p. 105, 1958.

Comments are closed.